Quality assurance in production: securing competitive advantages
Functioning quality assurance in production is essential for effective company operations. But what does quality assurance mean anyway? How is it different from quality control? And what is the significance of a LIMS (Laboratory Information Management System) for effective quality assurance? This is what we want to shed light on in this article. Securing competitive advantages with quality assurance in production.
Quality Assurance Definition: What is Quality Assurance?
To properly classify the term, it is important to understand the concept of quality management, abbreviated QM. The definition reads:
“All the activities of the overall management that, within the framework of the QM system, define the quality policy, objectives and responsibilities, as well as realize them through such means as quality planning, quality control, quality assurance/QM presentation and quality improvement.”
Quality assurance, abbreviated QA, is nothing more than the practical implementation of a company’s quality policy. Specifically, it is organizational and technical activities to ensure product quality. The term quality assurance is applied to all technical and organizational measures that serve to meet defined quality requirements in production and service provision. In this way, a specific quality is ensured at the earliest possible stage. According to the definition of the DIN EN ISO 9000:2015 standard, quality assurance is a subarea of quality management. All qualitative specifications and guidelines are defined by the company itself in each case. But of course, certain quality standards are also demanded by customers or determined by legal regulations and other provisions.
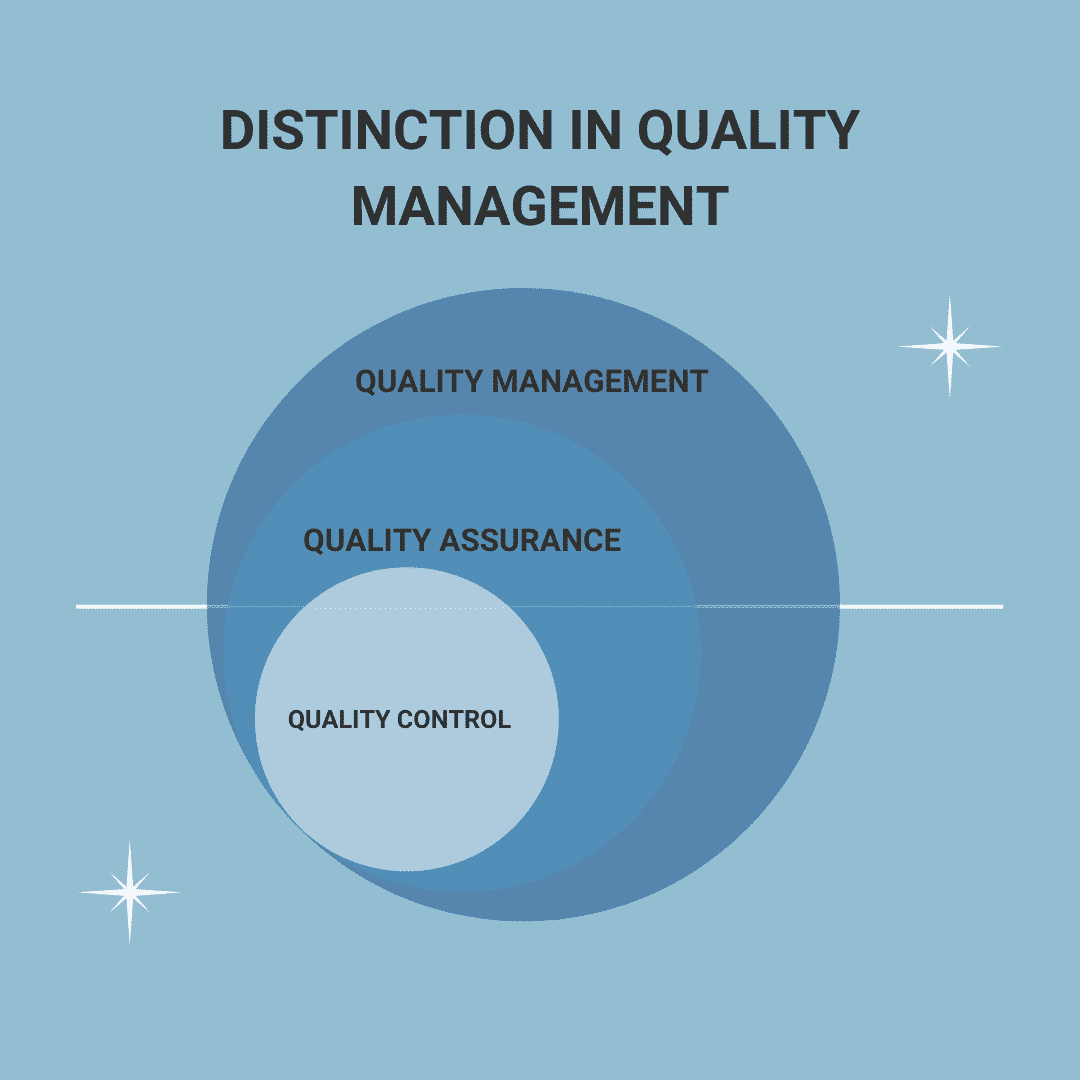
Difference quality assurance and quality control
In relation to quality assurance in production, the question often arises, “What is the difference between quality control and quality assurance?” Both terms, quality assurance and quality control, can be defined as sub-areas of quality management. The main difference between the terms is in the tasks: In quality assurance, the quality of the end product is ensured on the basis of defined specifications from quality management. The essential component is therefore the planning of how high-quality products can be produced. Quality control, on the other hand, starts later: Quality control identifies defects in the finished products and helps to correct them before delivery to the customer.
Why quality assurance? What are the goals of quality assurance?
There are several reasons why it is worthwhile to strive for professional quality assurance in production.
- Increasing quality = higher customer satisfaction: Quality is demonstrably an important decision criterion when choosing a product. Thus, targeted quality assurance increases customer satisfaction.
- Increase revenue: Enthusiastic customers buy more than dissatisfied customers. Professional quality assurance helps to expand the potential of existing customers and win new customers.
- Reduce costs: Transparently documented processes make it possible to uncover and exploit optimization potential. In this way, costs can be saved in the long term.
- Risk minimization: Consistently implemented quality management minimizes the risk of product liability and warranty claims.
- More efficient process organization: Optimized processes ensure that throughput times (e.g. for inquiries or complaints) are reduced. Duplication of work is avoided.

Quality assurance tasks
With regard to the trust placed in a company by customers, suppliers, employees and the public, the quality of the products or services is an essential factor. Quality assurance and its tasks do not start with production, but already during the development of products and services. Since the elimination of defects is often associated with high costs, quality assurance assumes particular importance at the early stage of production, because the elimination of defects at this point is usually associated with the lowest cost and time expenditure.

The tasks of quality assurance at a glance:
- Definition and implementation of systematic test concepts
- Creation of production control plans
- Ensuring product and service quality by monitoring process and workflows
- Ensuring and monitoring the proper execution of tests and inspections
- Training of employees
- Compliance with customer-specific requirements
- Processing external complaints
- Carrying out complaint management with suppliers
- Customer communication on quality-related issues
- Preparation of initial sample inspection reports
Internal quality assurance in the laboratory: Quality in laboratory diagnostics
For laboratories in manufacturing companies in all industries, quality assurance takes on a special significance. Measured values are constantly produced – the measuring methods used in the laboratory must pass a series of tests every day. The measured values produced must therefore be constantly tested for correctness and precision in the course of quality control. However, this quality requirement is often in conflict with the objective that laboratories should also work as economically and resource-friendly as possible. But how can you measure as efficiently and economically as possible and ensure quality standards if the manual acquisition of measurement data is so time-consuming?
Quality assurance in production with modern LIMS software
The solution: An automated, data-based, networked LIMS (Laboratory Information Management Software) makes it possible to create continuous quality awareness down to the smallest process step and to permanently ensure optimum measurement quality. A LIMS stores all the data that is generated in your laboratory on a daily basis. It supports you by automating data exchange and fully integrating all instruments. This ensures transparent tracking of your samples throughout the entire processing cycle and guarantees ideal quality management. Evaluations and reports on all data available in the system complete the functionalities.
Our LIMS software solution [FP]-LIMS is also an important step in the modernization of workflows or laboratory processes, as it minimizes paperwork and enables data to be stored in a digital environment. No matter which version you choose, our LIMS ensures higher efficiency, a reduction in the error rate (thus for better quality management), it reduces the documentation effort, and enables cost savings.