LIMS in use: Excerpt of our enthusiastic customers
Your advantages thanks to our modern LIMS
Extensive functions
[FP]-LIMS offers extensive functionalities even in the light version. The LIMS database is optimally tailored to the requirements of operating laboratories and convinces across the board.
Impressive flexibility
Are you looking for a LIMS with a high degree of flexibility? You have found it with [FP]-LIMS. It adapts completely to your individual business requirements – not the other way around.
Optimal price performance
We are proud to offer you a highly functional LIMS system at an unbeatable price-performance ratio. That’s why we communicate our prices quite transparently.
Proven laboratory solution
[FP]-LIMS software has been developed based on over 30 years of experience in performing, tracking, validating, and documenting measurements, and has been continuously enhanced with user feedback.
Worldwide awareness
More than 6000 installations around the world – international customers from numerous industries and with different focuses have relied on the proven cross-industrial [FP]–LIMS system, in some cases for decades.
We speak your language!
We are true laboratory process experts and know what LIMS must offer to optimally support your individual laboratory processes. This is reflected both in the functionalities of the software and in the terminology used.
LIMS solutions for a wide range of applications
The modern LIMS is used in many different application areas. It always unfolds its full power where a large amount of data has to be collected, analyzed, documented, and tracked. Find out below, for example, about the industry solutions or company size.

Standalone
Thanks to our [FP]-LIMS Light Version, the use of professional laboratory software is no longer reserved for large companies with the corresponding financial resources. The cost-effective version is a real help for small laboratories in particular in completing daily laboratory tasks. [FP]-LIMS Light is perfect for single users with one measuring device. This is how the LIMS system works today!

SMEs
[FP]-LIMS makes the everyday work of small and medium-sized companies much easier. By automating processes, you save a lot of time and reduce the risk of errors. The intelligent workflow accompanies you in your everyday tasks. Depending on the desired range of functions, [FP]-LIMS Standard or [FP]-LIMS Professional is recommended for SMEs. Secure your head start in the lab with the LIMS system.

Group
Thanks to our highly flexible LIMS system, the parallel use of different stand-alone solutions are no longer necessary. Handle your entire laboratory processes across the group with just one software. The high level of automation and intuitive workflows with central access for all laboratories ensure transparency across the group and a reduction in effort and costs.
The 8 biggest obstacles for testing labs to long-term quality assurance success
– Do you know these obstacles? –
You have an audit deviation
Functional LIMS software is often an important prerequisite for passing an audit. Consequences are a drop in sales and loss of orders.
Your lab grows - and so do your requirements
You want to scale healthily, but your Excel list is reaching its limits. You finally want to work centrally with multiple employees and become multi-client capable.
Your database is unreliable
Until now, you have mainly entered your data manually via Excel or Access. The process of data creation is no longer comprehensible; manual entry leads to errors. You would finally like to receive a more professional evaluation of your quality.
Simple laboratory processes are real time wasters
Simple, repetitive processes rob your employees of valuable time that you would rather invest in other topics. Creating a certificate takes hours, even manually entering measurement data eats up a lot of time. Surely there must be a simpler way?!
Trends you do not recognize at all or by chance
Professional evaluations are currently not possible at all or only with a lot of effort. You long for a reliable database with integrity, so that you can create detailed evaluations quickly and discover trends and dependencies at an early stage.
Your Excel expert leaves the company
Several years ago, a clever man built up your Excel list with all the macros and functionalities you need for your daily laboratory work. Now this expert is leaving the company – and with him the know-how.
Your current LIMS is inflexible
Your individual processes can only be transferred to your current LIMS with a lot of effort. You finally want to work with a LIMS that adapts to your processes and not vice versa.
Digitization? So far, nothing!
Digitization and automation of processes were previously foreign words in your company. You have recognized the relevance, would finally like to work more effectively, become more competitive and raise your quality to the next level.
You may have experienced in the past how such obstacles stand in the way of your long-term success in quality assurance.
Who benefits from the use of our LIMS?
– The best results in laboratory management –

Laboratory staff
Less effort
The use of [FP]-LIMS has been proven to reduce your documentation effort by up to 80%. Finally, more time for the main tasks!
More accurate results.
The [FP]-LIMS helps generate precise, reproducible results.
Full integration
Connect all lab and measurement devices to the [FP]–LIMS to create a fully digital environment for your lab management.

Quality Manager
Assurance of quality standards.
The [FP]-LIMS enables complete workflow automation, reducing human error.
Retraceability
A secure and reportable audit trail allows tracking of all data in the system and logs all changes.
Analyses and Evaluations
Extensive, automatable analyses and evaluations enable you to recognize trends in the measurements at an early stage.

CEOs
Reduce costs
The use of [FP]-LIMS reduces effort and eliminates paperwork. For you, this means immense cost savings!
Promote transparency
Receive automatically all important figures, data, and facts around the measurements in your laboratory and be always informed!
Integration into the IT system.
Whether ERP, MES, or PPS – We can be fully integrated into your production and business systems.
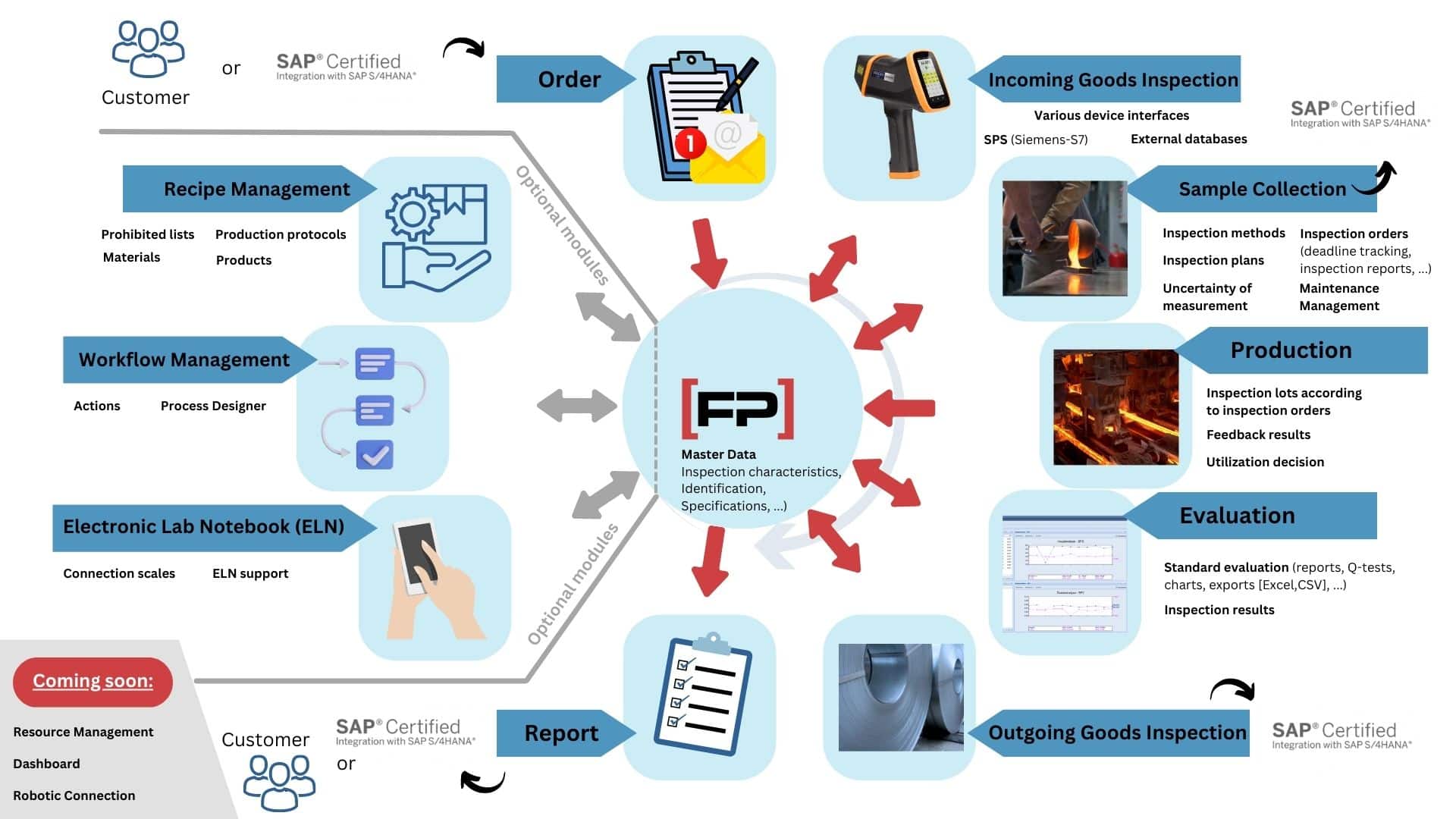
Our LIMS: FP-LIMS modules at a glance
– The right solution for every requirement –
Analysis Management
Manage analysis data professionally and document it securely – that’s what [FP]-LIMS Analysis Management offers you.
Inspection Order Management
Manage test orders for complex individual tests professionally – this is made possible by the [FP]-LIMS inspection order management.
Recipe Management
Manage your largest company assets in a secure database and safeguard your know-how.
ICP Module
Process and evaluate measurement data from ICP devices professionally – this is possible with the [FP]-LIMS ICP module.
Input Dialogue
Manual measurement data can be recorded more easily, making everyday work easier – this is possible with the [FP]-LIMS input dialogue.
ERP Module
The [FP]-LIMS ERP module ensures uncomplicated integration of the LIMS into the quality management process.
Browser Interface
Making relevant laboratory information easily available to other stakeholders within the company – that’s the idea behind the [FP]-LIMS Browser Interface.
Workflow Management System
Central control of laboratory-related business processes such as approvals or complaints procedures – that is the idea behind the Workflow Management System.
Frequently asked questions / FAQ
– Still questions about LIMS? Here you will find the answers –
Does FP-LIMS fit my company?
If you are looking for a powerful and flexible LIMS system, then you are like our thousands of customers worldwide from a wide range of industries who are already using [FP]-LIMS.
Our customers agree: [FP]-LIMS convinces above all with its high flexibility. But also a clear structure and comprehensive features even in the smallest version are more than convincing characteristics. Above all, we want the software to be highly practical in use, which is why it is constantly being further developed and optimized by our team of developers.
Make sure you arrange a free demo appointment, in which we will clarify your individual requirements and give you an insight into the software.
For which application areas is FP-LIMS suitable?
What our customers worldwide appreciate about our LIMS system is its high flexibility. This is also a major unique selling point.
This is evident both in the software itself (many features and parameters can be adapted independently and flexibly by the user), but also in the flexibility of the application.
Our [FP]-LIMS laboratory software is so flexibly designed that it can be used in a wide variety of industries. It is also shown in its flexibility when it comes to different sizes: From the smallest laboratory to the largest laboratory complex in a corporate group, you can use LIMS software. You also don’t need to worry about the interface connected to your measuring instruments – a variety of interfaces to many well-known measuring instruments are included as standard.
How much does the FP-LIMS system cost?
We are very proud that we can offer a powerful LIMS system at a very competitive price. In concrete terms, this means that our [FP]-LIMS Light (suitable for one user with the integration of a measuring device) is available for as little as $ 3,600 – so LIMS prices are very attractive. But also all other versions for more demanding tasks are comparatively inexpensive. You will be amazed!
By the way: Unless you decide to sign a service contract, all our prices are fixed prices. So you invest once and enjoy your powerful LIMS for a long time.
I already use a LIMS, but would like to change. What do I have to do now?
Our experienced team has already mastered moves to [FP]-LIMS for various customers in different industries. Experience has shown that the simple migration of data from external systems to [FP]-LIMS keeps the effort involved in the move within limits.
But you don’t have to worry: Our experts in our support team will accompany you throughout the entire process. We will be happy to analyze the current situation in a personal meeting and explain the options to you. However, we are happy to take on any challenge for you.
![[FP]-LIMS Software](https://fp-lims.com/wp-content/uploads/2020/06/Fink-Partner-LIMS-Software-Firmen-Logo-e1592470427959-300x250.png)